Have you ever wondered if it’s possible for water to boil and freeze at the same time? It sounds like a strange contradiction, but in the world of physics, it’s not only possible but an incredible demonstration of a phenomenon known as the Triple Point of water. Understanding this concept allows us to dive deeper into the behaviors of matter and how different states of water—solid, liquid, and gas—can coexist under specific conditions.
In this blog post, we’ll explore the Triple Point of water, explain the conditions under which water can boil and freeze simultaneously, and discuss why this phenomenon challenges our usual understanding of how liquids behave. Let’s dive into the fascinating world of physics and discover how water can boil and freeze at the same time!
What is the Triple Point?
The Triple Point refers to the unique set of conditions where all three phases of a substance—solid, liquid, and gas—can coexist in thermodynamic equilibrium. This phenomenon occurs at a very specific temperature and pressure. For water, the Triple Point happens at a temperature of 0.01°C (273.16 K) and a pressure of about 611.657 pascals. Under these conditions, water can exist simultaneously as ice (solid), water (liquid), and steam (gas).
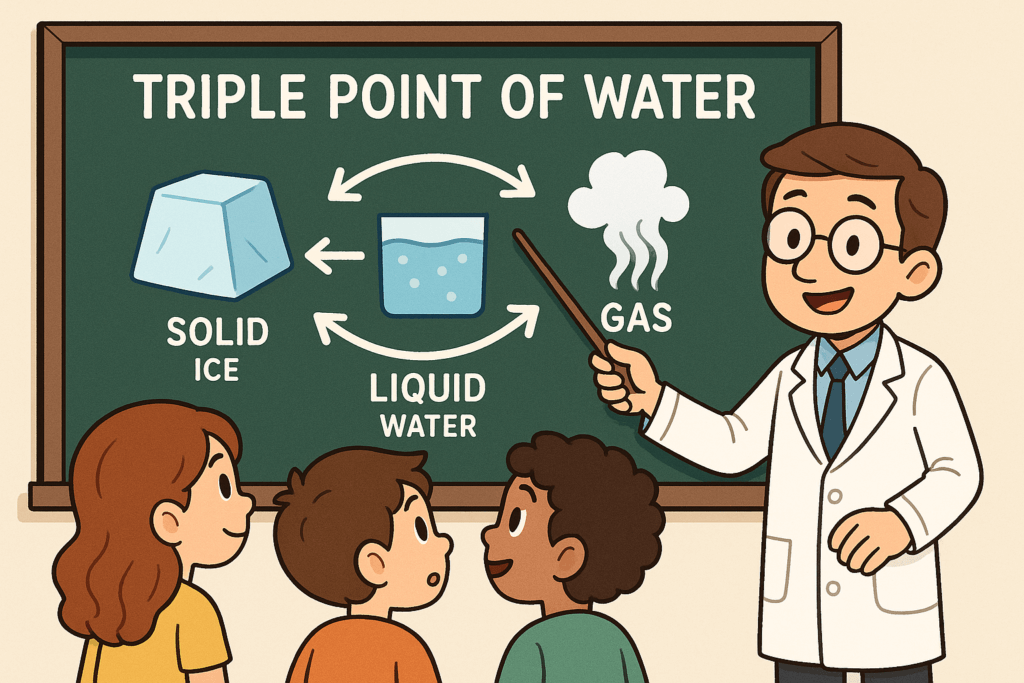
In simpler terms, the Triple Point of water is the moment when water exists as solid ice, liquid water, and gas (steam) all at the same time. You can think of it as a special intersection where all three states merge together, which, under regular circumstances, seems impossible. It is important to note that achieving the Triple Point requires very precise conditions of temperature and pressure, making it a rare and fascinating occurrence.
How Can Water Boil and Freeze at the Same Time?
To understand how water can boil and freeze simultaneously, we must first understand how boiling and freezing occur. Freezing happens when a liquid’s temperature drops below its freezing point, causing it to turn into a solid. Boiling occurs when the liquid’s temperature rises to its boiling point, causing it to transition into a gas.
So how can water do both at the same time? The answer lies in the Triple Point. When water is at its Triple Point, the temperature and pressure are perfectly balanced to allow water molecules to simultaneously transition into a solid (freeze) and into a gas (boil). At this exact point, water’s molecules have enough energy to form gas bubbles while also being able to form ice crystals. It is a delicate balance where all three states coexist, and it’s one of the most visually captivating phenomena in thermodynamics.
Thunder Moon: 5 Fascinating Facts About This Week’s Rare Moon
This happens because the temperature and pressure are in such a specific range that the usual distinctions between the phases of matter blur. So while water boils and freezes simultaneously at this temperature and pressure, the conditions are so delicate that it is not easy to observe outside of controlled experiments, such as in specialized labs or scientific demonstrations.
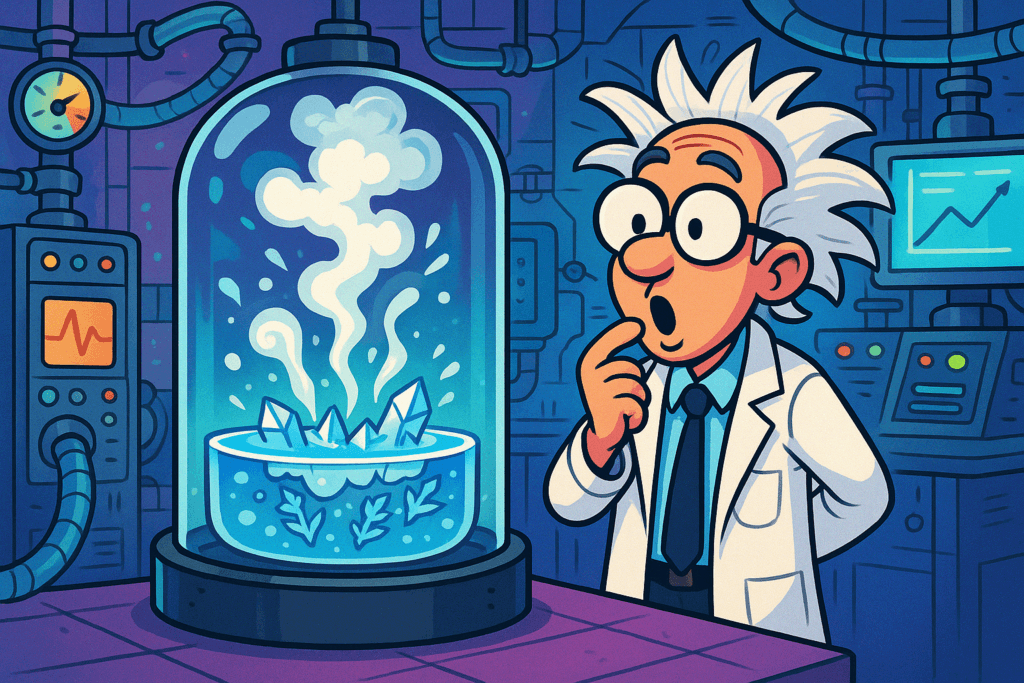
The Science Behind the Triple Point
When water reaches its Triple Point, it is technically not in a single state of matter. Instead, it exists in a kind of equilibrium, where the processes of freezing and boiling are balanced. This means that as water freezes into ice, it is simultaneously boiling into steam, all while maintaining a delicate equilibrium between the solid, liquid, and gas phases.
This phenomenon can be observed more easily in a lab setting under vacuum conditions. Since the pressure at the Triple Point is extremely low, it is often necessary to control the environment precisely to observe the boiling and freezing process. This is why the Triple Point is used by scientists and researchers to define the temperature and pressure scale for other substances as well, making it a foundational concept in thermodynamics.
Applications of the Triple Point Concept
The concept of the Triple Point is not just a quirky scientific fact; it has real-world applications in various fields of science and industry. One of the most critical uses of the Triple Point is in the calibration of thermometers. Since the Triple Point of water is such a precisely defined condition, it serves as a reference point for temperature measurements across different scientific fields.
In addition to this, the Triple Point concept is crucial in areas such as:
- Phase Diagram Studies: The Triple Point helps scientists understand and map out phase diagrams, which describe how a substance behaves under various conditions of pressure and temperature.
- Cryogenics and Low-Temperature Physics: The study of the Triple Point is fundamental in fields like cryogenics, where scientists study materials at extremely low temperatures to develop better cooling systems and technologies.
- Space Science: The Triple Point also plays a role in understanding the behavior of water and other substances in space, where temperature and pressure conditions can vary drastically.
Can the Triple Point Be Achieved on Earth?
Achieving the Triple Point of water on Earth under normal conditions is incredibly challenging due to the specific pressure and temperature required. However, it is achievable in controlled laboratory conditions. Scientists use specialized equipment, such as high-pressure chambers, to create the perfect environment needed to reach the Triple Point.
Though it may not be something we encounter every day, understanding the Triple Point concept allows scientists to gain deeper insights into the physical properties of substances. By studying these properties, researchers can develop new materials, improve temperature control systems, and even explore the behavior of substances in outer space.
Conclusion
The ability of water to boil and freeze at the same time is a fascinating concept that challenges our everyday understanding of physics. At the Triple Point, water exists in all three phases—solid, liquid, and gas—simultaneously, making it one of the most remarkable phenomena in thermodynamics. By studying the Triple Point of water, scientists can explore the properties of materials under extreme conditions, opening the door to new scientific discoveries and technological advancements.
Whether you’re a science enthusiast or simply curious about the strange wonders of our world, the Triple Point offers a perfect example of how nature can defy our expectations in the most intriguing ways.
Frequently Asked Questions (FAQ)
- What is the Triple Point of water?
The Triple Point of water is the unique set of conditions where water can exist simultaneously as a solid, liquid, and gas. It occurs at a temperature of 0.01°C and a pressure of 611.657 pascals. - Can water boil and freeze at the same time?
Yes, under specific conditions of temperature and pressure, such as at the Triple Point, water can simultaneously freeze into ice and boil into steam. - What temperature is the Triple Point of water?
The Triple Point of water occurs at 0.01°C (273.16 K). - Why is the Triple Point important?
The Triple Point is essential in scientific studies because it serves as a standard reference point for temperature and pressure measurements and plays a crucial role in fields like thermodynamics, cryogenics, and space science. - Where can the Triple Point of water be observed?
The Triple Point of water can be observed in specialized lab settings where temperature and pressure conditions can be precisely controlled.
